
Chemistry, 30.08.2019 17:20 carrietaylor234
For the following equilibrium: 3a + b = 20 if equilibrium concentrations are [a] = 1.1 m and [b] = 1.4 m, and kc = 11.3, what is the equilibrium concentration of c? • your answer should have two significant figures. provide your answer below:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
For the following equilibrium: 3a + b = 20 if equilibrium concentrations are [a] = 1.1 m and [b] =...
Questions



Computers and Technology, 27.06.2020 20:01






Computers and Technology, 27.06.2020 20:01



History, 27.06.2020 20:01

Business, 27.06.2020 20:01

English, 27.06.2020 20:01






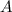 at equilibrium = 1.1 M
at equilibrium = 1.1 M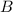 at equilibrium = 1.4 M
at equilibrium = 1.4 M
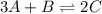
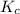 will be,
will be,![K_c=\frac{[C]^2}{[A]^3[B]}](/tpl/images/0212/4597/0cfd6.png)
![11.3=\frac{[C]^2}{(1.1)^3\times (1.4)}](/tpl/images/0212/4597/67486.png)
![[C]=4.6M](/tpl/images/0212/4597/2aa7c.png)


