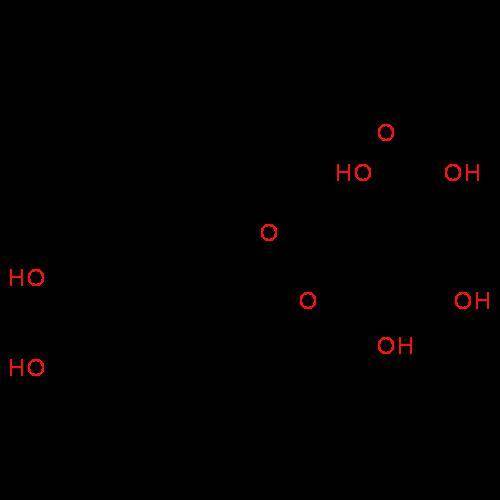
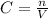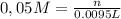Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an indicator solution and titrates with sodium hydroxide. he finds the equivalence point occurs when he's titrated 9.5 ml of 0.05 m sodium hydroxide. approximately how much chlorogenic acid is in the sample? 475 umol ® 480 umol © 500 umol 510 umol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3
You know the right answer?
Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an in...
Questions

Mathematics, 23.10.2019 16:50



History, 23.10.2019 16:50

English, 23.10.2019 16:50

History, 23.10.2019 16:50


Mathematics, 23.10.2019 16:50

Mathematics, 23.10.2019 16:50




Mathematics, 23.10.2019 16:50



Mathematics, 23.10.2019 16:50

History, 23.10.2019 16:50

Biology, 23.10.2019 16:50


Mathematics, 23.10.2019 16:50