
Chemistry, 30.08.2019 04:10 malasyamcclendon
3cu(s) + 8 hno3(aq) + 3 cu(no3)2(aq) + 2no(9) + 4h20() c) if 5.58 g of copper(ii) nitrate, cu(no3)2, is eventually obtained, how many moles of nitric acid, hno3, were used in the experiment?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
3cu(s) + 8 hno3(aq) + 3 cu(no3)2(aq) + 2no(9) + 4h20() c) if 5.58 g of copper(ii) nitrate, cu(no3)2,...
Questions





Mathematics, 31.07.2019 15:20

Mathematics, 31.07.2019 15:20

Mathematics, 31.07.2019 15:20


Mathematics, 31.07.2019 15:20





Mathematics, 31.07.2019 15:20

Mathematics, 31.07.2019 15:20

Mathematics, 31.07.2019 15:20




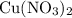 :
: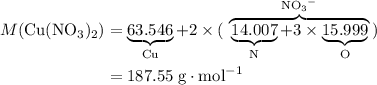 .
.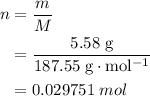 .
.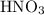 and that of
and that of 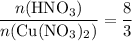 .
.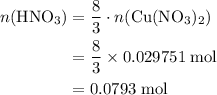 .
.

