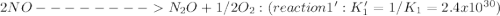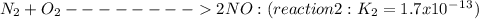Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x 10-13 find the value of the equilibrium constant for the following equilibrium reaction: n2(g) 1/202(g) n20(g) a) 7.0 x 10-44 b) 4.2 x 1017 c) 2.4 x 10-18 d) 1.6 x 109 e) 2.6 x 10-22

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Given the following: i) n20(g) 1/2 02(g) 2 no(g) ii) n2(g) 02(g) 2 no(g) ke= 4.1 x 10-31 ke= 1.7 x...
Questions

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00





Biology, 15.04.2021 01:00


Business, 15.04.2021 01:00


History, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00



Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00


Chemistry, 15.04.2021 01:00