

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3


Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
For a galvanic cell that uses the following two half-reactions, cr 2o 7 2-( aq) 14 h ( aq) 6 e - → 2...
Questions





Chemistry, 20.11.2019 02:31

Mathematics, 20.11.2019 02:31

Mathematics, 20.11.2019 02:31

Mathematics, 20.11.2019 02:31

Mathematics, 20.11.2019 02:31

Mathematics, 20.11.2019 02:31

Mathematics, 20.11.2019 02:31


Mathematics, 20.11.2019 02:31


History, 20.11.2019 02:31

Spanish, 20.11.2019 02:31

English, 20.11.2019 02:31


History, 20.11.2019 02:31

Mathematics, 20.11.2019 02:31

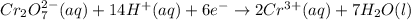
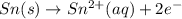
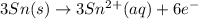
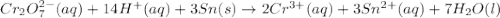
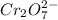 will oxidizes 3 moles of Sn
will oxidizes 3 moles of Sn moles of Sn
moles of Sn

