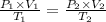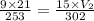Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2


Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
If a gas is initially at a pressure of 9 atm, a volume of 21 liters, and a temperature of 253 k, and...
Questions




History, 20.08.2019 06:30


English, 20.08.2019 06:30



Mathematics, 20.08.2019 06:30

Chemistry, 20.08.2019 06:30




Health, 20.08.2019 06:30

Mathematics, 20.08.2019 06:30



Mathematics, 20.08.2019 06:30

Biology, 20.08.2019 06:30