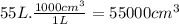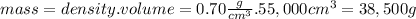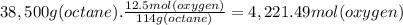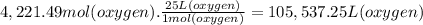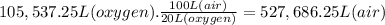Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
What volume of air is needed to burn an entire 55-l (approximately 15-gal) tank of gasoline? assume...
Questions

Mathematics, 14.01.2020 19:31

History, 14.01.2020 19:31




Arts, 14.01.2020 19:31


Biology, 14.01.2020 19:31






English, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31

History, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31