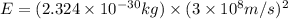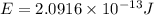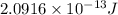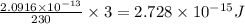Chemistry, 30.08.2019 02:20 emmadivaburnsox7ae9
How much energy is released by the decay of 3 grams of 230th in the following reaction 230 th - 226ra + "he (230 th = 229.9837 g/mol, 226ra = 225.9771 g/mol, "he = 4.008 g/mol) (10 pts.)

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
How much energy is released by the decay of 3 grams of 230th in the following reaction 230 th - 226r...
Questions

Biology, 22.10.2021 22:50




Chemistry, 22.10.2021 22:50


Mathematics, 22.10.2021 22:50



History, 22.10.2021 22:50



History, 22.10.2021 22:50

Social Studies, 22.10.2021 22:50

Arts, 22.10.2021 22:50

Business, 22.10.2021 22:50


Mathematics, 22.10.2021 22:50


English, 22.10.2021 22:50

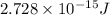
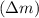 .
.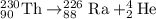
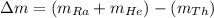
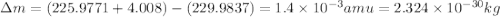
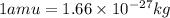 )
)