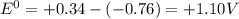Chemistry, 30.08.2019 00:30 mixcolin0002
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc sulfate. explain why there will be no reaction when zinc metal and aqueous copper sulfate solution are combined. identify the anode and the cathode, assuming a voltaic cell is constructed. note: be careful in the calculation of the standard cell potential ( eo cathode - eo anode). do not change the sign of the given reduction potential. the sign is already taken care of using the formula for calculating the standard cell potential.
standard reduction potential: cu2+(aq) + 2e- > cu(s) eo = -0.34 v
zn2+(aq) + 2e- > zn(s) eo = -0.76 v
standard cell potential = eo cathode - eo anode
although , combination of the reactants do not result in voltaic cells, it can be predicted if the reaction is spontaneous, based on the standard reduction potential.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

You know the right answer?
it is expected that a chemical reaction will occur when copper metal is combined with aqueous zinc s...
Questions

Biology, 22.02.2020 16:37

Computers and Technology, 22.02.2020 16:37

Mathematics, 22.02.2020 16:37

Mathematics, 22.02.2020 16:37

Mathematics, 22.02.2020 16:38


Biology, 22.02.2020 16:39


English, 22.02.2020 16:41



Mathematics, 22.02.2020 16:45


English, 22.02.2020 16:51

World Languages, 22.02.2020 16:51


English, 22.02.2020 16:54


Mathematics, 22.02.2020 16:58

Mathematics, 22.02.2020 16:58

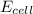 = +ve, reaction is spontaneous
= +ve, reaction is spontaneous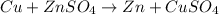
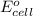 = standard electrode potential =
= standard electrode potential =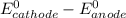
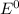 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Cu^{2+}/Cu]}= +0.34V](/tpl/images/0210/3859/55543.png)
![E^0_{[Zn^{2+}/Zn]}= -0.76V](/tpl/images/0210/3859/6c8c3.png)
![E^0=E^0_{[Zn^{2+}/Zn]}- E^0_{[Cu^{2+}/Cu]}](/tpl/images/0210/3859/f1898.png)
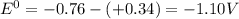
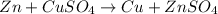
![E^0=E^0_{[Cu^{2+}/Cu]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0210/3859/cb03b.png)