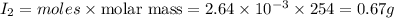Chemistry, 29.08.2019 22:30 tannersclark
Iodine reacts with aqueous thiosulfate ion in neutral solution according to the balanced equation 12(aq) + 25,03 -(aq) s4062(aq) + 27- (aq) how many grams of 12 are present in a solution if 35.20 ml of 0.150 m na2s2o, solution is needed to titrate the la solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Iodine reacts with aqueous thiosulfate ion in neutral solution according to the balanced equation 12...
Questions



English, 12.05.2021 23:00



Social Studies, 12.05.2021 23:00


Mathematics, 12.05.2021 23:00



Mathematics, 12.05.2021 23:00


Chemistry, 12.05.2021 23:00


Health, 12.05.2021 23:00

Mathematics, 12.05.2021 23:00


Mathematics, 12.05.2021 23:00

Mathematics, 12.05.2021 23:00

History, 12.05.2021 23:00


![moles of [tex]Na_2S_2O_3=Molarity\times {\text {Volume in L}}=0.150\times 0.0352=5.28\times 10^{-3}moles](/tpl/images/0210/1117/e01d3.png)

 require 1 mole of
require 1 mole of 
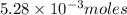 require=
require=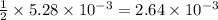 moles of
moles of