break down the following question in a step by step easy to comprehend process.
when one atom...

break down the following question in a step by step easy to comprehend process.
when one atom of iodine-131 decays by beta particle emission, 1.5541 x 10^ -33 j are released. the atomic mass of the product nuclide is 130.9051 atomic mass units. write a nuclear reaction for the decay of iodine-131 and determine its atomic mass in amu. 1 amu = 1.66053886 x 10^ -27kg; mass of a b particle = 9.10938291 x 10^ -31kg

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
Questions

Biology, 15.07.2019 09:30

History, 15.07.2019 09:30

Mathematics, 15.07.2019 09:30

Mathematics, 15.07.2019 09:30

Biology, 15.07.2019 09:30

Mathematics, 15.07.2019 09:30

Mathematics, 15.07.2019 09:30

Mathematics, 15.07.2019 09:30

History, 15.07.2019 09:30


Mathematics, 15.07.2019 09:30

Health, 15.07.2019 09:30

Health, 15.07.2019 09:30

Mathematics, 15.07.2019 09:30

English, 15.07.2019 09:30





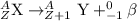
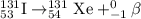

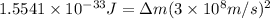
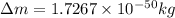
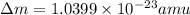
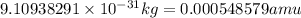
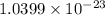 amu
amu

