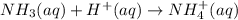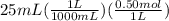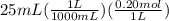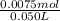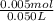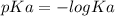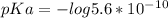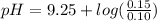Chemistry, 29.08.2019 20:00 cupkakekawaii45
Abuffer is prepared by combining 25 ml of 0.5o mnh(aq) with 25 ml of 0.20 m hci. what is the ph of the buffer? (ka (nh4) = 5.6 x 10 ) a. 8.86 b. 9.65 c. 8.00 d. 7.76 e. 9.43

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


You know the right answer?
Abuffer is prepared by combining 25 ml of 0.5o mnh(aq) with 25 ml of 0.20 m hci. what is the ph of t...
Questions


Mathematics, 29.11.2019 08:31




Health, 29.11.2019 08:31


History, 29.11.2019 08:31

English, 29.11.2019 08:31


Mathematics, 29.11.2019 08:31

Mathematics, 29.11.2019 08:31

Physics, 29.11.2019 08:31

Mathematics, 29.11.2019 08:31

History, 29.11.2019 08:31

Mathematics, 29.11.2019 08:31

Mathematics, 29.11.2019 08:31


English, 29.11.2019 08:31

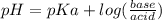
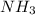 which is a weak base. 25 mL of 0.20 M HCl, a strong acid are added. Ammonia reacts with HCl to form its conjugate acid, ammonium ion. The net ionic equation will be:
which is a weak base. 25 mL of 0.20 M HCl, a strong acid are added. Ammonia reacts with HCl to form its conjugate acid, ammonium ion. The net ionic equation will be: