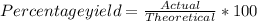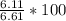Chemistry, 29.08.2019 19:10 yazanadel56
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide. ca) + h2o --> ca(oh)2 in a particular experiment, a 5.00 g sample of cao is reacted with excess water and 6.11 g of ca(oh)2 is recovered. what is the percent yield in this experiment?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide. ca) + h2o --...
Questions

Mathematics, 11.01.2021 07:30

Mathematics, 11.01.2021 07:30

History, 11.01.2021 07:30

Mathematics, 11.01.2021 07:30

Chemistry, 11.01.2021 07:30

Chemistry, 11.01.2021 07:30

Chemistry, 11.01.2021 07:30

Mathematics, 11.01.2021 07:30

Social Studies, 11.01.2021 07:30





Mathematics, 11.01.2021 07:30

Mathematics, 11.01.2021 07:30



Mathematics, 11.01.2021 07:30


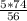 = 6.61 g of Ca(OH)₂.
= 6.61 g of Ca(OH)₂.