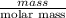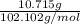Chemistry, 29.08.2019 19:10 sarmientojose267
Four beakers containing potassium nitrate dissolved in water are allowed to evaporate to dryness. beakers 1 through 4 contain 2.3, 1.91, 5.985, and 0.52 g of dry potassium nitrate respectively. how many moles of potassium nitrate were recovered after the water evaporated?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Four beakers containing potassium nitrate dissolved in water are allowed to evaporate to dryness. be...
Questions





Social Studies, 10.04.2020 00:17




Spanish, 10.04.2020 00:17

Mathematics, 10.04.2020 00:17

Social Studies, 10.04.2020 00:17


Geography, 10.04.2020 00:17







Geography, 10.04.2020 00:17

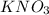 = molar mass of K + molar mass of N + 3 × molar mass of O
= molar mass of K + molar mass of N + 3 × molar mass of O