
Chemistry, 29.08.2019 17:30 cheychey021203
Calculate the hydroxide ion concentration in an aqueous solution with a ph of 4.33 at 25°c. a) 2.1 * 10-10 m b) 9.7 * 10-10 m c) 4.7 x 10-5 m d) 3.8 x 10-5 m

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2
You know the right answer?
Calculate the hydroxide ion concentration in an aqueous solution with a ph of 4.33 at 25°c. a) 2.1 *...
Questions

Social Studies, 26.07.2019 17:30

Biology, 26.07.2019 17:30

Social Studies, 26.07.2019 17:30

Geography, 26.07.2019 17:30






Computers and Technology, 26.07.2019 17:30

Arts, 26.07.2019 17:30

Physics, 26.07.2019 17:30

English, 26.07.2019 17:30


Biology, 26.07.2019 17:30

Computers and Technology, 26.07.2019 17:30

Biology, 26.07.2019 17:30

Chemistry, 26.07.2019 17:30


Computers and Technology, 26.07.2019 17:30

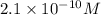
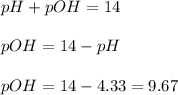
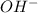 concentration.
concentration.![pOH=-\log [OH^-]](/tpl/images/0209/3278/1fac1.png)
![9.67=-\log [OH^-]](/tpl/images/0209/3278/ead14.png)
![[OH^-]=2.1\times 10^{-10}M](/tpl/images/0209/3278/1c94c.png)


