
Chemistry, 29.08.2019 01:20 emmapizano
Use the following, balanced equation for the question below: 2al(s) + 3cucl2(aq) → 3cu(s) + 2alcl3(aq) how many moles of copper metal will be produced from 10.0 moles of aluminum metal? 10.0 moles of copper 15.0 moles of copper 20.0 moles of copper 30.0 moles of copper

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

You know the right answer?
Use the following, balanced equation for the question below: 2al(s) + 3cucl2(aq) → 3cu(s) + 2alcl3(...
Questions


History, 20.08.2020 18:01

Social Studies, 20.08.2020 18:01

Social Studies, 20.08.2020 18:01

Mathematics, 20.08.2020 18:01

Mathematics, 20.08.2020 18:01

Mathematics, 20.08.2020 18:01



Business, 20.08.2020 18:01






Social Studies, 20.08.2020 18:01

Biology, 20.08.2020 18:01

History, 20.08.2020 18:01

Mathematics, 20.08.2020 18:01

Mathematics, 20.08.2020 18:01


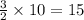 moles of copper.
moles of copper.

