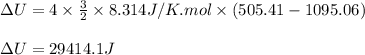Chemistry, 28.08.2019 23:00 michaellangley
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas (cv = 3/2 r). the compression reduces the volume of the gas from 0.26 m^3 to 0.12 m^3. the change in the internal energy of the gas, in kj is ("^3" means to the power of 3)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas...
Questions

Mathematics, 06.05.2021 19:10

English, 06.05.2021 19:10

Mathematics, 06.05.2021 19:10

Business, 06.05.2021 19:10

Mathematics, 06.05.2021 19:10

Mathematics, 06.05.2021 19:10

Mathematics, 06.05.2021 19:10



Mathematics, 06.05.2021 19:10

English, 06.05.2021 19:10

Computers and Technology, 06.05.2021 19:10

English, 06.05.2021 19:10


Mathematics, 06.05.2021 19:10





Biology, 06.05.2021 19:20

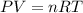
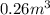
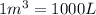
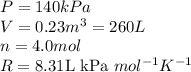
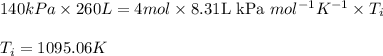
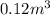
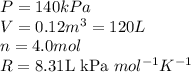
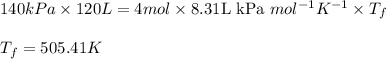
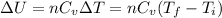
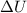 = change in internal energy = ?
= change in internal energy = ?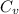 = heat capacity at constant volume =
= heat capacity at constant volume = 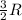
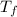 = final temperature = 1095.06 K
= final temperature = 1095.06 K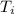 = initial temperature = 505.41 K
= initial temperature = 505.41 K