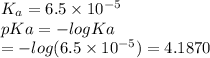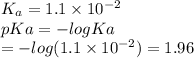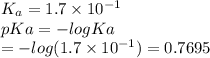Chemistry, 28.08.2019 21:30 Andrewecolt1993
Which of the following acids (listed with ka values) and their conjugate base would form a buffer with a ph of 2.34? a. hf, ka = 3.5 x 10-4 b. c6h5cooh, ka = 6.5 x 10-5 c. hclo2, ka = 1.1 x 10-2 d. hclo, ka = 2.9 x 10-8 e. hio3, ka = 1.7 x 10-1

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Which of the following acids (listed with ka values) and their conjugate base would form a buffer wi...
Questions



History, 04.09.2020 14:01



Mathematics, 04.09.2020 14:01



Geography, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01


Mathematics, 04.09.2020 14:01


Mathematics, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01

Business, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01