Chemistry, 28.08.2019 21:10 heavenwagner
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone and phenol in the chemical industry. combustion of 47.6 mg cumene produces some co2 and 42.8 mg water. the molar mass of cumene is between 115 and 125 g/mol. determine the empirical and molecular formulas.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

You know the right answer?
Cumene is a compound containing only carbon and hydrogen that is used in the production of acetone a...
Questions


Mathematics, 23.04.2020 21:02


Mathematics, 23.04.2020 21:02






Social Studies, 23.04.2020 21:02






Health, 23.04.2020 21:03


Chemistry, 23.04.2020 21:03


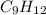
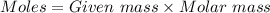
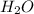 = 42.8/18 = 2.3778×10⁻³ moles
= 42.8/18 = 2.3778×10⁻³ moles