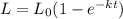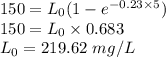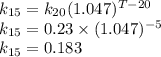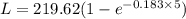Chemistry, 28.08.2019 21:00 QueenNerdy889
Some wastewater has a bod5 of 150 mg/l at 20 o c. nitrification was inhibited. the reaction rate k at that temperature has been determined to be 0.23 /day. (a) find the ultimate carbonaceous bod. (b) find the reaction rate coefficient at 15 o c. (c) find the bod5 at 15 o c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
Some wastewater has a bod5 of 150 mg/l at 20 o c. nitrification was inhibited. the reaction rate k a...
Questions

Chemistry, 10.04.2020 21:55

Mathematics, 10.04.2020 21:55








Mathematics, 10.04.2020 21:55

Mathematics, 10.04.2020 21:55







Mathematics, 10.04.2020 21:55