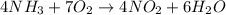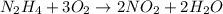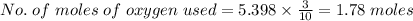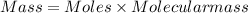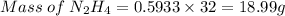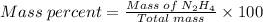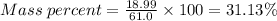Agas contains a mixture of nh3(g) and n2h4(g), both of which react with o2(g) to form no2(g) and h2o(g). the gaseous mixture (with an initial mass of 61.00 g) is reacted with 10.00 moles o2, and after the reaction is complete, 4.062 moles o2 remains. calculate themass percent of n2h4(g) in the original gaseous mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Agas contains a mixture of nh3(g) and n2h4(g), both of which react with o2(g) to form no2(g) and h2o...
Questions

Mathematics, 21.06.2019 18:00






Mathematics, 21.06.2019 18:00




Mathematics, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00





History, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00


Mathematics, 21.06.2019 18:00