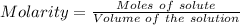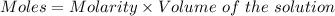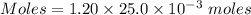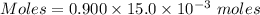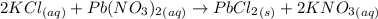Chemistry, 28.08.2019 17:30 leomessifanboy678
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii) nitrate solution and this precipitation reaction occurs: 2 kcl(aq)+pb(no3)2(aq)→pbcl2(s)+2 kno3(aq) the solid pbcl2 is collected, dried, and found to have a mass of 2.45 g. determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii)...
Questions


Social Studies, 30.04.2021 23:40






Health, 30.04.2021 23:40

Mathematics, 30.04.2021 23:40

Mathematics, 30.04.2021 23:40

Chemistry, 30.04.2021 23:40


Mathematics, 30.04.2021 23:40



Mathematics, 30.04.2021 23:40

Mathematics, 30.04.2021 23:40

Mathematics, 30.04.2021 23:40


Mathematics, 30.04.2021 23:40