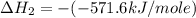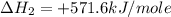Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

You know the right answer?
Given that δh = −571.6 kj/mol for the reaction 2 h2(g) + o2(g) → 2 h2o(l), calculate δh for these re...
Questions


English, 21.10.2021 01:00

History, 21.10.2021 01:00

Mathematics, 21.10.2021 01:00



English, 21.10.2021 01:00

Biology, 21.10.2021 01:00








Mathematics, 21.10.2021 01:00




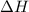 for the reaction is +571.6 kJ/mole.
for the reaction is +571.6 kJ/mole.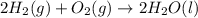
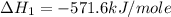
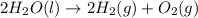
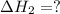
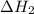 for the reaction will be:
for the reaction will be: