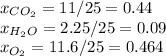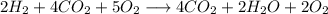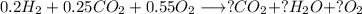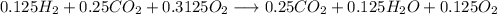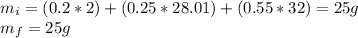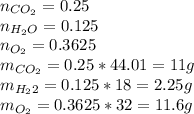Chemistry, 28.08.2019 16:20 itsyagirlgona21
Agas mixture with the molar analysis 20% h2, 25% co, 55% o2 reacts to form products consisting of co2, h2o, and o2 only. determine the amount of each product, in kg per kg of mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

You know the right answer?
Agas mixture with the molar analysis 20% h2, 25% co, 55% o2 reacts to form products consisting of co...
Questions







Computers and Technology, 16.12.2019 21:31





History, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31




Mathematics, 16.12.2019 21:31



English, 16.12.2019 21:31