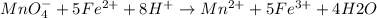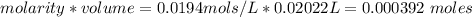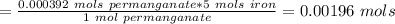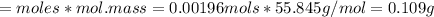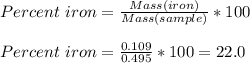Chemistry, 28.08.2019 05:20 kayleenfifep3hgi4
A0.495 g sample of iron containing salt required 20.22 ml of 0.0194 m permanganate solution to reach the endpointof a titration. what is the percent of iron (55.845 g/mol) in the salt

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
A0.495 g sample of iron containing salt required 20.22 ml of 0.0194 m permanganate solution to reach...
Questions


Mathematics, 05.06.2021 01:00

Mathematics, 05.06.2021 01:00

Physics, 05.06.2021 01:00

Social Studies, 05.06.2021 01:00

Mathematics, 05.06.2021 01:00


Mathematics, 05.06.2021 01:00


Mathematics, 05.06.2021 01:00


Mathematics, 05.06.2021 01:00

Mathematics, 05.06.2021 01:00

Mathematics, 05.06.2021 01:00


Mathematics, 05.06.2021 01:00




Mathematics, 05.06.2021 01:00