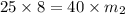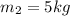Chemistry, 28.08.2019 04:30 kentavonwilliam
How many kilograms of water must evaporate from 8kg of a 25% salt solution to produce 40% salt solution?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3

Chemistry, 23.06.2019 08:00
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1
You know the right answer?
How many kilograms of water must evaporate from 8kg of a 25% salt solution to produce 40% salt solut...
Questions

Mathematics, 30.10.2020 20:30

Mathematics, 30.10.2020 20:30

Mathematics, 30.10.2020 20:30

English, 30.10.2020 20:30

Mathematics, 30.10.2020 20:30



Mathematics, 30.10.2020 20:30

Biology, 30.10.2020 20:30

History, 30.10.2020 20:30

Mathematics, 30.10.2020 20:30


History, 30.10.2020 20:30


Mathematics, 30.10.2020 20:30




Mathematics, 30.10.2020 20:30

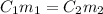
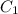 = concentration of ist solution = 25%
= concentration of ist solution = 25%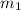 = mass of ist solution = 8 kg
= mass of ist solution = 8 kg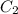 = concentration of second solution = 40%
= concentration of second solution = 40%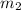 = mass of second solution = ? kg
= mass of second solution = ? kg