
Chemistry, 27.08.2019 18:30 corbinfisher
Potassium chromate is slowly added to a solution containing 0.20 m agno3 and 0.20 m ba(no3)2. describe what happens if the ksp for ag2cro4 is 1.1 × 10-12 and the ksp of bacro4 is 1.2 × 10-10.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Potassium chromate is slowly added to a solution containing 0.20 m agno3 and 0.20 m ba(no3)2. descri...
Questions

History, 17.07.2019 16:50


Mathematics, 17.07.2019 16:50

History, 17.07.2019 16:50


Computers and Technology, 17.07.2019 16:50

Chemistry, 17.07.2019 16:50

Physics, 17.07.2019 16:50

Mathematics, 17.07.2019 16:50


Mathematics, 17.07.2019 16:50

Mathematics, 17.07.2019 16:50

Social Studies, 17.07.2019 16:50

Social Studies, 17.07.2019 16:50

Mathematics, 17.07.2019 16:50





Health, 17.07.2019 16:50

![[CrO_4^{-2}] or [K_2CrO_4]=2.75 \times10^{-11} M](/tpl/images/0203/1152/d4689.png)
![[CrO_4^{-2}] or [K_2CrO_4]=6.0\times10^{-10} M](/tpl/images/0203/1152/0eea9.png)
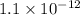
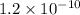
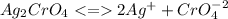
![Ksp = [Ag^{+}]^{2}[CrO_4^{-2}]=1.1\times10^{-12}](/tpl/images/0203/1152/3bbe1.png)
![(0.20)^{2} \times [CrO_4^{-2}]=1.1\times10^{-12}](/tpl/images/0203/1152/5e50f.png)
![[CrO_4^{-2}]=2.75 \times10^{-11} M](/tpl/images/0203/1152/0bd03.png)
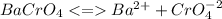
![Ksp = [Ba^{2+}][CrO_4^{-2}]=1.2\times10^{-10}](/tpl/images/0203/1152/b6fad.png)
![(0.20) \times [CrO_4^{-2}]=1.2\times10^{-10}](/tpl/images/0203/1152/289af.png)
![[CrO_4^{-2}]=6.0 \times10^{-10} M](/tpl/images/0203/1152/a9e96.png)


