

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3


Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
When 0.42 g of a compound containing c, h, and o is burned completely, the products are 1.03 g co2 a...
Questions

Mathematics, 09.11.2020 20:10

Physics, 09.11.2020 20:10


Biology, 09.11.2020 20:10

Social Studies, 09.11.2020 20:10


Mathematics, 09.11.2020 20:10

Social Studies, 09.11.2020 20:10




Mathematics, 09.11.2020 20:10

SAT, 09.11.2020 20:10

History, 09.11.2020 20:10


Physics, 09.11.2020 20:10

Computers and Technology, 09.11.2020 20:10


English, 09.11.2020 20:10

History, 09.11.2020 20:10

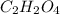 and
and 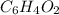
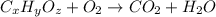
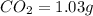
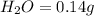
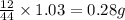 of carbon will be contained.
of carbon will be contained.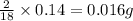 of hydrogen will be contained.
of hydrogen will be contained.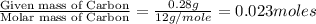
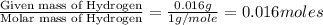
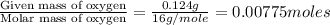
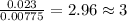
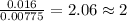
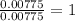
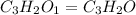
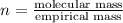
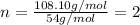
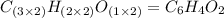
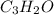 and
and 


