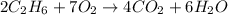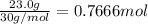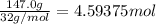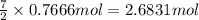Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 23. g of ethane is mixed with 147. g of oxygen. calculate the minimum mass of ethane that could be left over by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions



Physics, 09.03.2021 22:00





Spanish, 09.03.2021 22:00

English, 09.03.2021 22:00

Mathematics, 09.03.2021 22:00


Mathematics, 09.03.2021 22:00

Mathematics, 09.03.2021 22:00

Mathematics, 09.03.2021 22:00

Mathematics, 09.03.2021 22:00


Spanish, 09.03.2021 22:00

Chemistry, 09.03.2021 22:00

Mathematics, 09.03.2021 22:00