Chemistry, 27.08.2019 17:20 siriuskitwilson9408
What is the change in entropy when 7.61 ml of liquid benzene (c6h6, d = 0.879 g/ml) is combusted in the presence of 22.3 l of oxygen gas, measured at 298 k and 1 atm pressure? (r = 0.0821 l · atm/(k · mol)) 2c6h6(l) + 15o2(g) → 12co2(g) + 6h2o(l); δs° = –437.7 j/k at 298 k a. 436 j/k b. 37.4 j/k c. 398 j/k d. 45.3 j/k e. 18.7 j/k

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
What is the change in entropy when 7.61 ml of liquid benzene (c6h6, d = 0.879 g/ml) is combusted in...
Questions

French, 16.02.2021 01:00

Mathematics, 16.02.2021 01:00


Chemistry, 16.02.2021 01:00

English, 16.02.2021 01:00







Mathematics, 16.02.2021 01:00



Mathematics, 16.02.2021 01:00

Mathematics, 16.02.2021 01:00

Chemistry, 16.02.2021 01:00

Advanced Placement (AP), 16.02.2021 01:00

Spanish, 16.02.2021 01:00

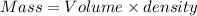
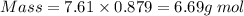
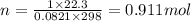
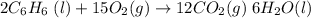
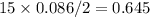 mol of O2
mol of O2