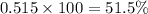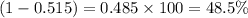The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x, one weighing 59 g/mol, and the other weighing 61 g/mol. what is the relative abundance of each?
possible answers:
50% x-59, 50% x-61
75% x-59, 25% x-61
63% x-59, 37% x-61
51.5% x-59, 48.5% x-61
48.5% x-59, 51.5% x-61

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
The average molecular weight for element x is 59.97 g/mol. there are two known isotopes of element x...
Questions

Mathematics, 16.07.2020 04:01




Mathematics, 16.07.2020 04:01



Social Studies, 16.07.2020 04:01



Mathematics, 16.07.2020 04:01









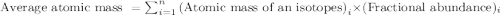 .....(1)
.....(1)![59.97 g/mol=[(59 g/mol\times x)+(61 g/mol\times (1-x))]\\\\x=0.515](/tpl/images/0202/9296/4c349.png)