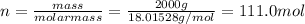Chemistry, 27.08.2019 17:10 Jackiebear4593
Assuming the mass of the ice in the beaker was 2000 g (2 kg), calculate the amount of heat that must have been added to convert the ice to water (assume that the entire 2000 g was ice at the start of melting, and the entire 2000 g was water at the end of the melting). assume that the change took place entirely at 0 °c. the heat of fusion (dhfus) for h2o is 6.12 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Assuming the mass of the ice in the beaker was 2000 g (2 kg), calculate the amount of heat that must...
Questions

History, 23.06.2021 19:10



Chemistry, 23.06.2021 19:10




Mathematics, 23.06.2021 19:10

Biology, 23.06.2021 19:10






Chemistry, 23.06.2021 19:10

Mathematics, 23.06.2021 19:10