
Chemistry, 27.08.2019 02:30 camballard3848
The freezing point of ethanol (c2h5oh) is -114.6 °c. the molal freezing point depression constant for ethanol is 2.00 °c/m. what is the freezing point (°c) of a solution prepared by dissolving 50.0 g of glycerin (c3h8o3, a nonelectrolyte) in 200 g of ethanol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
The freezing point of ethanol (c2h5oh) is -114.6 °c. the molal freezing point depression constant fo...
Questions

Mathematics, 23.02.2022 14:00

Advanced Placement (AP), 23.02.2022 14:00


SAT, 23.02.2022 14:00


Mathematics, 23.02.2022 14:00







Physics, 23.02.2022 14:00

Geography, 23.02.2022 14:00

SAT, 23.02.2022 14:00





SAT, 23.02.2022 14:00

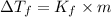
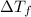 = Depression in freezing point
= Depression in freezing point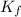 = Molal freezing point depression constant
= Molal freezing point depression constant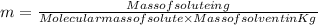
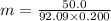
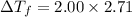 = 5.42 °C
= 5.42 °C

