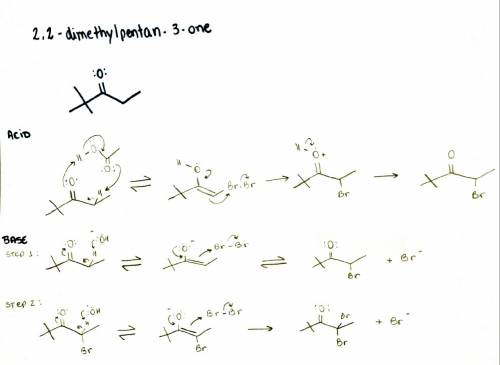
The reaction of 2,2-dimethylpentan-3-one with bromine yields a monobrominated product in acidic medium but gives a dibrominated product in basic medium. draw the products of each reaction and propose an explanation as to why there is a difference in reactivity between acidic and basic media.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

You know the right answer?
The reaction of 2,2-dimethylpentan-3-one with bromine yields a monobrominated product in acidic medi...
Questions


Mathematics, 20.11.2020 23:50

History, 20.11.2020 23:50

Biology, 20.11.2020 23:50

Mathematics, 20.11.2020 23:50

History, 20.11.2020 23:50



Mathematics, 20.11.2020 23:50

Geography, 20.11.2020 23:50

Mathematics, 20.11.2020 23:50


Mathematics, 20.11.2020 23:50


Advanced Placement (AP), 20.11.2020 23:50


German, 20.11.2020 23:50



Mathematics, 20.11.2020 23:50