
Sulfur dioxide gas reacts with sodium hydroxide to form sodium sulfite and water. the unbalanced chemical equation for this reaction is given below: so2(g) + naoh(s) → na2so3(s) + h2o(l)assuming that you start with 36.8 g of sulfur dioxide and 20.7 g of sodium hydroxide and assuming that the reaction goes to completion, determine the mass of each product formed. g na2so3g h2o

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1
You know the right answer?
Sulfur dioxide gas reacts with sodium hydroxide to form sodium sulfite and water. the unbalanced che...
Questions

Mathematics, 12.12.2020 17:50



History, 12.12.2020 17:50




Mathematics, 12.12.2020 17:50



Mathematics, 12.12.2020 17:50

Mathematics, 12.12.2020 17:50

Medicine, 12.12.2020 17:50

Advanced Placement (AP), 12.12.2020 17:50




Spanish, 12.12.2020 18:00


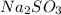 and 9.3 g of
and 9.3 g of  were formed
were formed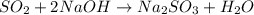
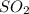 = 64 g
= 64 g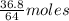 of
of 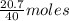 of NaOH = 0.5175 moles of NaOH
of NaOH = 0.5175 moles of NaOH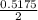 moles of
moles of 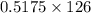 ) g = 65.2 g
) g = 65.2 g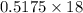 ) g = 9.3 g
) g = 9.3 g

