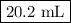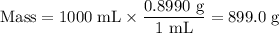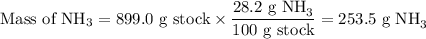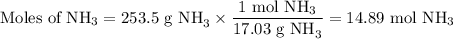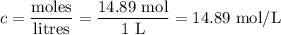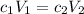Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Astock solution is 28.2 percent ammonia (nh3) by mass, and the solution has a density of 0.8990 gram...
Questions




History, 25.11.2020 20:50


Biology, 25.11.2020 20:50

Arts, 25.11.2020 20:50



Mathematics, 25.11.2020 20:50

Social Studies, 25.11.2020 20:50

Arts, 25.11.2020 20:50

English, 25.11.2020 20:50

Business, 25.11.2020 20:50




Biology, 25.11.2020 20:50

English, 25.11.2020 20:50