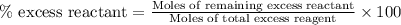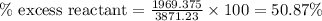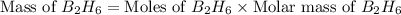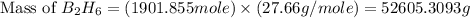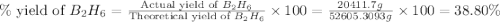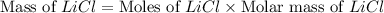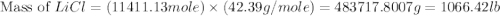Diborane, b2h6 a possible rocket propellant, can be made by using lithium hydride (lih): 6 lih+ 2 bcl2àb2h6+ 6 licl . if you mix 200 lb of lih with 1000 lb of bcl3 , you recover 45 lb of b2h6. determine (a) limiting reactant (b) the excess reactant (c) the percent excess reactant (d) the percent conversion of lih to b2h6 (e) lb of licl produced

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 11:30
How many grams of carbon are in 237 grams of ethanol(c2h5oh) and how many sulfide ions are in 2.45 moles of aluminum sulfide show me you you got the answers
Answers: 3
You know the right answer?
Diborane, b2h6 a possible rocket propellant, can be made by using lithium hydride (lih): 6 lih+ 2 b...
Questions




















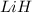
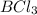
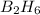 or percent conversion of
or percent conversion of 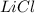 produced is, 1066.42 lb
produced is, 1066.42 lb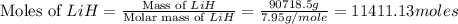
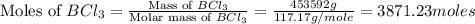
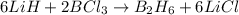
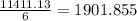 moles of
moles of 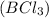 .
.