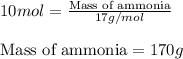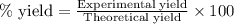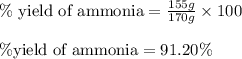Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g) + 3h2 (g) 2nh3 (g). if 140 grams of nitrogen gas is allowed to react with an excess of hydrogen gas to produce 155 grams of ammonia, what is the percent yield of this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
Ammonia gas is formed from nitrogen gas and hydrogen gas, according to the following equation, n2 (g...
Questions






Chemistry, 25.07.2019 06:00

Biology, 25.07.2019 06:00


History, 25.07.2019 06:00







Social Studies, 25.07.2019 06:00


Social Studies, 25.07.2019 06:00

Chemistry, 25.07.2019 06:00

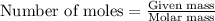
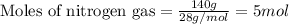
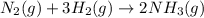
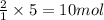 of ammonia.
of ammonia.