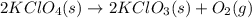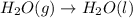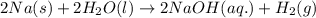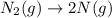Chemistry, 23.08.2019 01:30 hallmansean04
4. without consulting appendix 3 in the textbook, predict whether the entropy change is positive or negative for each of the following reactions. give reasons for your predictions. (12 points) a. 2kclo4(s) → 2kclo3(s) + o2(g) b. h2o(g) → h2o(l) c. 2na(s) + 2h2o(l) → 2naoh(aq) + h2(g) d. n2(g) → 2n(g)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
4. without consulting appendix 3 in the textbook, predict whether the entropy change is positive or...
Questions

Chemistry, 28.12.2019 02:31



History, 28.12.2019 02:31


Mathematics, 28.12.2019 02:31



Health, 28.12.2019 02:31


English, 28.12.2019 02:31

Mathematics, 28.12.2019 02:31

English, 28.12.2019 02:31


Mathematics, 28.12.2019 02:31

Mathematics, 28.12.2019 02:31




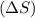 . Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
. Randomness of gaseous particles is more than that of liquid which is further more than that of solids.