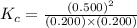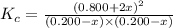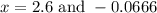Chemistry, 23.08.2019 01:10 ahoney2233
At equilibrium, the concentrations in this system were found to be [n21 o20.200 m and [no]0.500 m. n2(8) 02e) 2no(g) if more no is added, bringing its concentration to 0.800 m, what will the final concentration of no be after equilibrium is re-established?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
At equilibrium, the concentrations in this system were found to be [n21 o20.200 m and [no]0.500 m. n...
Questions


Mathematics, 22.10.2020 20:01



English, 22.10.2020 20:01


Geography, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01


Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

Computers and Technology, 22.10.2020 20:01

History, 22.10.2020 20:01

History, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

Mathematics, 22.10.2020 20:01

World Languages, 22.10.2020 20:01


 at equilibrium is 0.9332 M
at equilibrium is 0.9332 M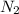 and
and 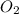 at equilibrium = 0.200 M
at equilibrium = 0.200 M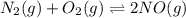
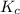 will be,
will be,![K_c=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0189/4962/71f8f.png)