
Ch4 and h2o were mixed in a 0.64 l reactor at 1800 k. steam reforming took place according to: ch4 (g) + h2o (9) co (g) + 3 h2 (9) the equilibrium constant for this reaction is k+0.28. at equilibrium, the reactor contained 0.36 mol of co, 0.081 mol of ha and 0.051 mol of ch. what is the concentration of h20 at equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1


Chemistry, 23.06.2019 16:20
Which of the following subject areas contains questions that can be answered by science? alchemy ethics forensics politics
Answers: 3
You know the right answer?
Ch4 and h2o were mixed in a 0.64 l reactor at 1800 k. steam reforming took place according to: ch4...
Questions


Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00

English, 15.04.2021 01:00



History, 15.04.2021 01:00






Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00

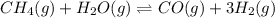

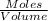
![[CO]=\frac{0.36 mol}{0.64 L}](/tpl/images/0189/2611/ede89.png)
![H_2=[H_2]=\frac{0.081 mol}{0.64 L}](/tpl/images/0189/2611/21859.png)
![CH_4=[CH_4]=\frac{0.051 mol}{0.64 L}](/tpl/images/0189/2611/7135e.png)
![H_2O=[H_2O]=?](/tpl/images/0189/2611/180f8.png)
![K_c=\frac{[CO][H_2]^3}{[CH_4][H_2O]}](/tpl/images/0189/2611/498b4.png)
![0.28=\frac{\frac{0.36 mol}{0.64 L}\times (\frac{0.081 mol}{0.64 L})^3}{\frac{0.051 mol}{0.64 L}\times [H_2O]}](/tpl/images/0189/2611/960b2.png)
![[H_2O]=0.05110 mol/L](/tpl/images/0189/2611/997c7.png)


