Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

You know the right answer?
Coke is an impure form of carbon that is often used in the in- dustrial production of metals from th...
Questions

Physics, 25.06.2019 21:30

Biology, 25.06.2019 21:30

History, 25.06.2019 21:30

Biology, 25.06.2019 21:30

Mathematics, 25.06.2019 21:30


Mathematics, 25.06.2019 21:30


Mathematics, 25.06.2019 21:30



Mathematics, 25.06.2019 21:30

History, 25.06.2019 21:30



Mathematics, 25.06.2019 21:30

Mathematics, 25.06.2019 21:30

Mathematics, 25.06.2019 21:30


History, 25.06.2019 21:30

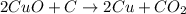
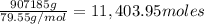
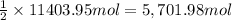 of carbon
of carbon