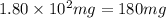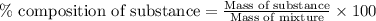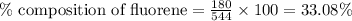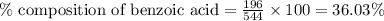A544 mg of a mixture of fluorene and benzoic acid was weighed out and subjected to an extraction and recrystallization. after this purification was completed the product crystals were dried and analyzed. the purification procedure produced 1.80 × 102 mg of fluorene and 196 mg of benzoic acid. calculate the percent composition of this mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1
You know the right answer?
A544 mg of a mixture of fluorene and benzoic acid was weighed out and subjected to an extraction and...
Questions

Mathematics, 07.07.2019 09:30

History, 07.07.2019 09:30

History, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30

Health, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30


Mathematics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30

English, 07.07.2019 09:30

English, 07.07.2019 09:30

English, 07.07.2019 09:30

Physics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30

Biology, 07.07.2019 09:30



Mathematics, 07.07.2019 09:30

Biology, 07.07.2019 09:30