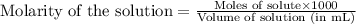Chemistry, 22.08.2019 20:30 ImGoodAtLife7797
Which of the following is true regarding the concentration of solutions?
a. percent solutions are parts per 1000 parts.
b. molarity is one mole of solute per 1000 ml of solution.
c. to calculate molarity, one must know the atomic weight of the solvent.
d. to calculate molarity, one must know the atomic number of the solute

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1


You know the right answer?
Which of the following is true regarding the concentration of solutions?
a. percent solution...
a. percent solution...
Questions


Mathematics, 03.06.2021 17:30


English, 03.06.2021 17:30

Computers and Technology, 03.06.2021 17:30


Mathematics, 03.06.2021 17:30

Mathematics, 03.06.2021 17:30

Mathematics, 03.06.2021 17:30

English, 03.06.2021 17:30

Spanish, 03.06.2021 17:30

Geography, 03.06.2021 17:30

Arts, 03.06.2021 17:30

Biology, 03.06.2021 17:30


History, 03.06.2021 17:40

Social Studies, 03.06.2021 17:40

Mathematics, 03.06.2021 17:40

Biology, 03.06.2021 17:40

Mathematics, 03.06.2021 17:40