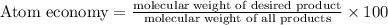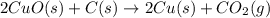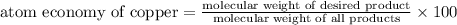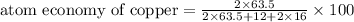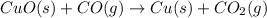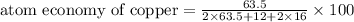Chemistry, 22.08.2019 19:10 GEEKLIFE6598
The following two reactions are possible methods for refining copper in the final step of a smelting process, i. e., getting pure copper (cu) from copper ores found in rocks. calculate the theoretical atom economy for each reaction. a. 2 cuo(s) + c(s) → 2 cu(s) + co2(g) = % b. cuo(s) + co(g) → cu(s) + co2(g) = %

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
The following two reactions are possible methods for refining copper in the final step of a smelting...
Questions

Computers and Technology, 04.02.2020 14:02


Mathematics, 04.02.2020 14:03

History, 04.02.2020 14:03

Mathematics, 04.02.2020 14:03




Social Studies, 04.02.2020 14:03



History, 04.02.2020 14:03




Mathematics, 04.02.2020 14:03


English, 04.02.2020 14:03

Computers and Technology, 04.02.2020 14:03