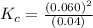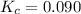Chemistry, 22.08.2019 18:30 bjpvrpow74wq
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350 mol of o2 and excess carbon were placed in a 5.00-l container and heated, the equilibrium concentration of co was found to be 0.060 m. what is the equilibrium constant, kc, for this reaction?
a. 0.001
b. 0.072
c. 0.090
d. 1.2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:40
How can you increase the ability of a gas to dissolve in a liquids?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 21:20
Juju.01) 5 geologic events a group of students designed an experiment in an ice rink to represent the solar system. the steps of the experiment are listed below. 5: geologic events 1. choose a student to represent the sun. the planets are represented by two tennis balls. 2. ask the student to hold the tennis balls in each palm and spin on the ice with arms stretched out. 3. ask the student to draw in the arms after about 10 spins. 4. observe the student's arms rotate faster when they are closer to the body. 05 geologic events enors) the experiment most likely demonstrates that (2 points) 07 discussion-based sessment/module planets exert gravitational force on the sun the sun exerts gravitational force on the planets 3.07 discussion-based ssessment speed of a planet depends on its distance from the sun new version available! (3.0.119) get it now submit 18.07: module exam description
Answers: 3

You know the right answer?
At high temperatures, carbon reacts with o2 to produce co as follows: c(s) o2(g) 2co(g). when 0.350...
Questions

Mathematics, 11.05.2021 04:00

Mathematics, 11.05.2021 04:00




English, 11.05.2021 04:00

History, 11.05.2021 04:00

Mathematics, 11.05.2021 04:00




Computers and Technology, 11.05.2021 04:00



Social Studies, 11.05.2021 04:00

Computers and Technology, 11.05.2021 04:00


Health, 11.05.2021 04:00

Mathematics, 11.05.2021 04:00

 = 0.350 mole
= 0.350 mole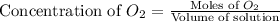
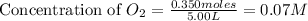
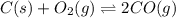
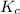 will be,
will be,![K_c=\frac{[CO]^2}{[O_2]}](/tpl/images/0188/5908/f4b67.png)
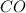 at equilibrium is, 0.060 M
at equilibrium is, 0.060 M