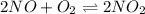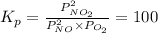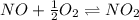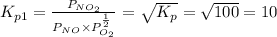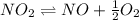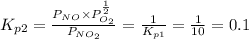Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

You know the right answer?
Find the equilibrium constants, kp, for the following equilibria, (i) no(g) + ½ o2(g) ⇄ no2(g), kp =...
Questions

Mathematics, 13.07.2019 05:00



Mathematics, 13.07.2019 05:00

Spanish, 13.07.2019 05:00


History, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00

Physics, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00


Physics, 13.07.2019 05:00


English, 13.07.2019 05:00

Mathematics, 13.07.2019 05:00


History, 13.07.2019 05:00

English, 13.07.2019 05:00

Physics, 13.07.2019 05:00