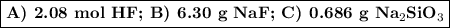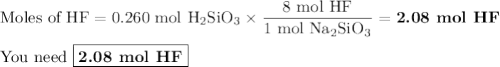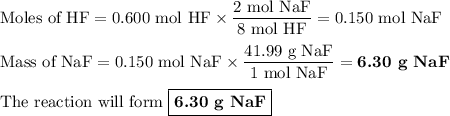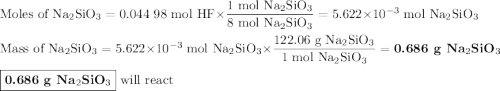Hydrofluoric acid, hf(aq), cannot be stored in glass bottles because compounds called silicates in the glass are attacked by the hf(aq). sodium silicate (na2sio3), for example, reacts as follows: na2sio3(s)+8hf(aq)→h2sif6(aq)+2naf( aq)+3h2o(l)? a)how many moles of hf are needed to react with 0.260 mol of na2sio3? b) how many grams of naf form when 0.600 mol of hf reacts with excess na2sio3? c)how many grams of na2sio3 can react with 0.900 g of hf?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Hydrofluoric acid, hf(aq), cannot be stored in glass bottles because compounds called silicates in t...
Questions

Mathematics, 08.02.2021 19:40

History, 08.02.2021 19:40


Arts, 08.02.2021 19:40


Computers and Technology, 08.02.2021 19:40

Mathematics, 08.02.2021 19:40

Mathematics, 08.02.2021 19:40

Mathematics, 08.02.2021 19:40



Mathematics, 08.02.2021 19:40






Geography, 08.02.2021 19:40

Mathematics, 08.02.2021 19:40

Medicine, 08.02.2021 19:40