
Chemistry, 22.08.2019 16:10 achsahjosey
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and magnesium is added to the acid. the reaction produces a soluble salt and hydrogen gas. 2.1 write a balanced equation for the reaction. 2.2 give the name of the soluble salt. 2.3 has a physical or chemical change taken place? 2.4 2 moles of hydrogen is produced. how many moles of acid reacted?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

You know the right answer?
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and...
Questions


History, 04.08.2019 19:20

Biology, 04.08.2019 19:20




Social Studies, 04.08.2019 19:20

Mathematics, 04.08.2019 19:20









Mathematics, 04.08.2019 19:20

Mathematics, 04.08.2019 19:20

History, 04.08.2019 19:20

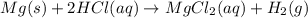
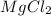
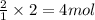 of HCl
of HCl

