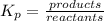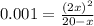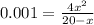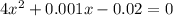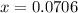Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the...

Chemistry, 22.08.2019 05:10 chrisraptorofficial
Consider the following gas phase chemical reaction:
a(g) -- > 2b(g)
write down the expression for the equilibrium constant of this reaction.
if the initial concentration of a is 20 atm pressure, the initial concentration of b is 0 atm and the equilibrium constant kp for the reaction is .001 atm-1, calculate the equilibrium concentration of b.
i know the first part of this would be kc = [a] / [b]2 i need the second part

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2


You know the right answer?
Questions



Mathematics, 06.03.2022 20:50

English, 06.03.2022 20:50



Mathematics, 06.03.2022 20:50


Mathematics, 06.03.2022 20:50

Chemistry, 06.03.2022 20:50




Mathematics, 06.03.2022 21:00

Mathematics, 06.03.2022 21:00


Chemistry, 06.03.2022 21:00

Chemistry, 06.03.2022 21:00

Mathematics, 06.03.2022 21:00

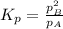 and equilibrium concentration of B is 0.141 atm.
and equilibrium concentration of B is 0.141 atm.